The New York Times :
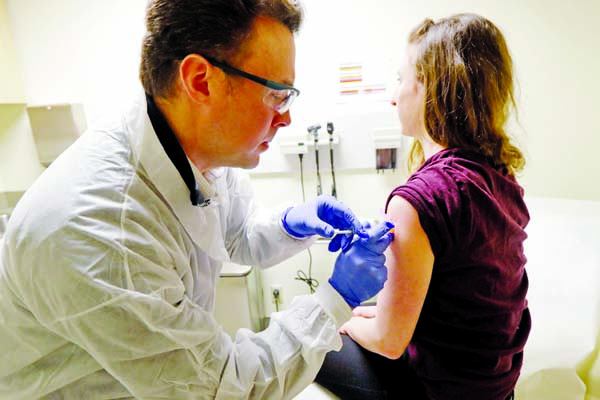
The first testing in humans of an experimental vaccine for the new coronavirus began on Monday, the National Institute of Allergy and Infectious Diseases announced.
The main goal of this first set of tests is to find out if the vaccine is safe. If it is, later studies will determine how well it works.
The trial was “launched in record speed,” Dr. Anthony Fauci, the institute’s director, said in a statement.
Such rapid development of a potential vaccine is unprecedented, and it was possible because researchers were able to use what they already knew about related coronaviruses that had caused other diseases outbreaks, SARS and MERS. Despite the rapid progress, even if the vaccine is proved safe and effective against the virus, it will not be available for at least a year.
The tests, which are being conducted at Kaiser Permanente Washington Health Research Institute in Seattle, use a vaccine made by Moderna Inc.
Seattle was chosen as a test site before the United States had any known coronavirus cases, not because of the outbreak that erupted there. Washington State has been hard hit by the virus, with more than 670 cases to date.
Get an informed guide to the global outbreak with our daily coronavirus newsletter.
Moderna uses genetic material – messenger RNA – to make vaccines, and the company has nine others in various stages of development, including several for viruses that cause respiratory illnesses. But no vaccine made with this technology has yet reached the market.
The infectious disease institute has been working with Moderna because the RNA approach can produce vaccine very quickly, said Dr. Barney Graham, the deputy director of the institute’s Vaccine Research Center.
He said the researchers at the vaccine center were focused on pandemic preparedness.
“The goal here is to be ready for all the virus families that can infect humans,” he said.
As bad as this epidemic is, Dr. Graham said, in one way it is lucky that a coronavirus caused it, because the researchers were at least partly ready for it. If another type of virus had caused the outbreak, it could have taken months longer to create a potential vaccine.
Other companies, using different approaches, are also trying to manufacture coronavirus vaccines. Moderna is the first to reach a clinical trial.
The trial will enroll 45 healthy adults ages 18 to 55. Each will receive two shots, 28 days apart. Moderna calls the vaccine mRNA-1273.